Siemens Simcenter STAR-CCM+ mimics the flow from an inhaler into the mouth and airway. Image courtesy of Siemens Digital Industries Software.
Latest News
January 6, 2021
It’s now more common than not for simulation and modeling tools to spearhead early R&D efforts to explore, iterate and refine medical device designs while also serving as the means to create cheaper virtual prototypes.
Less common, but equally promising, are nascent efforts to employ those same tools later in the process as a way to validate and test medical device prototype behavior in different patient scenarios for efficiency and safety.
The next chapter is augmenting and potentially replacing expensive and lengthy animal and human clinical trials with simulation and modeling initiatives with an eye toward reducing costs, accelerating time-to-market cycles and boosting quality as manufacturers commercialize innovative device classes.
The prevalence of chronic diseases, underscored by the havoc wreaked this year by the COVID-19 global pandemic, is mounting pressure on societies, government and health care systems to innovate bolder, more patient-specific solutions more quickly than in the past. This push for heightened design innovation is tempered by the reality of skyrocketing development and clinical trial costs, which can make the journey of launching new devices increasingly unsustainable.
That’s where simulation and modeling come into play. The technologies are already helping medical device manufacturers explore more design possibilities virtually, at less cost than building physical prototypes.
New simulation modalities and multiphysics capabilities, coupled with the accessibility of high-performance cloud computing horsepower are also paving the way for CAE and computational modeling to be leveraged as part of testing and validation, streamlining a multi-years process and ensuring products get to market on an accelerated timeline.
“The main benefit [of simulation-led workflows] is time—you develop a better device that performs better for the patient and is safer because you have a better understanding of what you are building,” notes Arlen Ward, principal at System Insight Engineering. “Simulation and modeling becomes a way to reduce some of the development costs. There is a role for simulation to play from the earliest ideation all the way through regulatory approval and commercialization.”
Though there is ample opportunity and heightened anticipation for how simulation and computational modeling can transform the complexities of clinical trials and help meet regulatory requirements, that leg of the journey is now just underway and challenges remain. For one, while the Food and Drug Administration (FDA) has actively given the green light to greater use of simulation and modeling throughout the medical device development process, there are gaps in available standards and the lack of codified guidelines remains a significant hurdle.
In addition, in contrast with industries such as aerospace and defense or automotive where simulation is employed throughout the end-to-end lifecycle, simulation in the medical device arena faces greater complexities due to the challenges of modeling biological tissue.
“Biological tissue is not a great material for modeling compared to aluminum or steel because it is so variable,” Ward explains. “There’s a lot of complexity that other industries didn’t have to deal with, but models have gotten better at mimicking tissues, and cloud computing and more powerful software is helping to tackle these difficult problems.”
Laying the Groundwork
Dassault Systèmes and its customer base have been at the forefront of simulation-based medical device development for a while. Beyond leveraging simulation and modeling to explore and optimize early design concepts, Dassault customers like Novo Nordisk have added simulation workflows to model polymer behaviors, for example, to fine-tune the latest configurations for products like an insulin pen, and to inform manufacturing processes to ensure safe and high-quality production.
More prominently, Dassault’s landmark Living Heart Project is at the epicenter of many groundbreaking initiatives, including exploring use of simulation for in silico testing and eventually, for clinical trials.
The Living Heart, powered by a collaborative ecosystem of researchers, medical device developers, regulatory agencies and practicing cardiologists, is an evolving collection of highly accurate and personalized digital human heart models designed as a foundation for in silico medicine and testing of medical device designs.
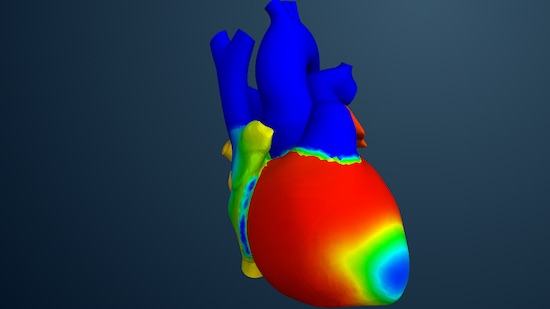
In the second phase of this effort, and in collaboration with the FDA, the initiative is working to make the 3D heart model a source of digital evidence for new cardiovascular device approvals, including as the basis for in silico clinical trials with the goal of reducing animal testing as well as the number of patients participants.
With the Living Heart models, it’s possible to run tests under different conditions as opposed to being constrained by a limited physical test bench.
“Instead of doing one or two runs, you can run hundreds or thousands of experiments,” says Karl D’Souza, Life Sciences Industry Solution Experience director, Dassault Systèmes.
Traditionally, medical device makers would have to create a lot of situations in the physical world to represent the variability of the human body, but the Living Heart enables them to understand how a device behaves in a multitude of hearts or in a single heart.
“Benchtop animal testing is costly, time-consuming and provides a piecemeal understanding,” D’Souza explains. “It can be augmented by something more rigorous that allows for exploration of possibilities both from a design and population perspective.”
Siemens Digital Industries Software’s Simcenter simulation portfolio is also helping medical device makers get closer to in silico testing.
Vyaire Medical, a maker of respiratory care equipment, is moving away from using simplified models for computational fluid dynamics (CFD) simulations of respiratory masks to more realistic and individualized simulations by introducing patient-specific data into those models.
By incorporating the lung structure and volume of various breathing profiles, the company is building a library of patient profiles and morphologies along with usage scenarios—for example, an otherwise healthy young adult with COVID-19 or elderly patient with lung disease—so it can simulate an entire spectrum of clinical usage, officials at Vyaire Medical say.
In fact, in silico studies have a distinct advantage over other forms of device evaluation as they allow for parametric, statistical and patient-specific analysis of a device’s performance in a highly controlled manner, according to Marc Horner, senior principal engineer for healthcare with Ansys.
Consider the example of an insulin pump, which is comprised of integrated systems of electronic, fluidic and mechanical components. Using Ansys’ Multiphysics simulation portfolio to perform full systems modeling, engineers could explore potential safety hazards of the device, including virtual kinking analysis via CFD to ensure there is no obstruction of insulin flow, finite element analysis to identify components that are prone to failure if dropped, as well as electromagnetics simulation to determine where to position an antenna for optimized performance.
Coupling those simulations with detailed models representing different classes of patients elevates the exercise to an entirely different level.
“We can form a population of patients and see if the insulin pump, as designed, will be able to deliver therapies to different classes of patients,” Horner explains. “In this way, we start to get a better understanding if the system works at a holistic level to treat these patients before we even go out to the field.”
Over the last few years, the FDA has taken significant steps to support the use of computational modeling and simulation in medical device applications. The Center for Devices and Radiological Health (CDRH) and the agency as a whole, has made simulation and modeling a priority and allowed results to be included as part of regulatory submissions, says Pras Pathmanathan, research scientist at CDRH and co-chair of the Modeling and Simulation Working group at the FDA.
The Modeling and Simulation Working group is also partnering in several initiatives, including Dassault’s Living Heart Project, and as part of a working group with the devices community, developed the ASME V&V 40 Standard, published in 2018, which demonstrates the reliability of modeling and simulation for medical devices.
Leveraging simulation to support in silico clinical trials is the next frontier, Pathmanathan says, although the discipline would augment, not replace, traditional trials, and there needs to be similar collaboration to develop guidelines, standards and best practices.
“In silico clinical trials could be performed in advance of a real trial to test whether the trial is expected to be successful or not,” he explains. “They could also be used to identify patient sub-populations that might respond to the proposed therapy allowing for better clinical trial design. Ending trials early, designing better trials or avoiding trials that would fail could all potentially save millions of dollars for industry and allow devices to reach the market faster while remaining safe and effective.”
Enabling In Silico Clinical Trials
BioMerieux and its BioFire subsidiary are fully invested in leveraging simulation and computational modeling to gain an in-depth understanding of its systems, which in turn will give them more confidence in what they ultimately take to clinical trials, according to officials.
Parent company BioMerieux has long tapped COMSOL simulation tools early in its process to optimize device designs through system modeling, while at BioFire, the practice comes into play in the post-release process to get a deeper understanding of complex systems to aid in making changes and supporting legacy systems.
Although the simulation and modeling work won’t serve as a replacement for physical clinical trials, it will shorten the process of pre-trial internal testing while ensuring a more polished product goes to trial.
“Modeling and simulation give you confidence in your system and your clinical trial results,” confirms Laurent Drazek, data science lead and in charge of sequencing & molecular at biotechnology firm BioMerieux.
Emphysys, Inc., which delivers product development services in the semiconductor and medical device fields, is actively using simulation and modeling to help clients design clinical trials with the goal of reducing costs and time, according to Daniel Smith, director of modeling and simulation for the firm, which is also a COMSOL certified partner.
Simulation can help predict the outcome of a particular procedure given a specific patient type, and the results can help reduce the field of patient types and scenarios that need to be tested with physical trials.
“For medical device manufacturers, simulation could ensure a shorter trial or a smaller trial,” adds John Carey, Emphysys’ head of business development. “It ends up helping them get to market faster while improving efficiency and safety.”
Nevertheless, there’s still a host of hurdles to overcome before simulation and computational modeling become a mainstream part of in silico and pre-clinical trial processes.
Among the most glaring gaps are the lack of codified best practices to support simulation and modeling practices for this use case along with the lack of skilled expertise, including engineers that are computational specialists with in-depth knowledge of biological and human body systems, experts say.
Ultimately, many existing hurdles can be chalked up to a maturity issue as simulation and modeling use in medical device development simply lags other industries.
“You can use modeling and simulation to determine if you are directionally correct or if something is a good idea conceptually, but not precisely,” says James Thompson, senior director of strategy and solutions for the medical device and pharmaceutical industries at Siemens Digital Industries. “When you’re talking about augmenting trials or doing in silico testing, close doesn’t count—you have to be precise, and that’s a maturity that’s currently lacking in the industry.”
Beth Stackpole is a contributing editor to DE. You can reach her at beth@digitaleng.news.
More Ansys Coverage
More COMSOL Coverage
More Dassault Systemes Coverage

More Siemens Digital Industries Software Coverage
Subscribe to our FREE magazine, FREE email newsletters or both!
Latest News
About the Author
Beth Stackpole is a contributing editor to Digital Engineering. Send e-mail about this article to DE-Editors@digitaleng.news.
Follow DE